HPC User Report from N. Vučemilović-Alagić (PULS group of the Physics Department)
Multiscale modelling of catalytic reactions in ionic liquid environment
Catalysis occurring in ionic liquids phase is an emerging ecologically friendly technology, currently revolutionizing a number of processes in chemical industry. However, the intelligent design of these systems is challenging and requires multi-scale simulations to set the foundations for future optimisation.
Motivation and problem definition
Structuring of the ionic liquids at liquid-gas and solid-liquid interfaces creates a unique dynamic environment for catalysis. However, there is no clear understanding of the structural and transport properties of functionalised ionic liquids and reactant molecules within the film. Furthermore, the coupling between the interface environment and the chemical transformations during catalysis are widely unexplored, an issue which we aim to rectify in the current projects.
Methods and codes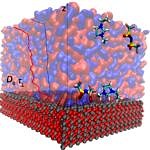
We combine quantum mechanics calculations for modeling chemical transformations (Gaussian16/Gaussview 6 and Turbomole), and molecular dynamics simulations to study structuring and transport (GROMACS, LAMMPS), each thoroughly validated against the wealth of experiments, to tune the functionality of the catalytic complex, the ionic liquids, and the support material.
Results
Building on our experience in modeling complex liquids [1-3] we provided a general and transferable model for simulations of nanoconfined ionic liquids [4]. This work on characterizing the physicochemical properties of ionic fluid films is complemented with the study of the water gas shift reaction in supported ionic liquid phase (SILP). After determining the active form of the catalyst [5], we were able to determine the reaction mechanism [6]. A similar strategy allowed us and our collaborators to understand the role of metal salt additives and to optimize the SILP composition towards enhancing the overall reaction turnover [7,8].
Outreach
This work provided preliminary results for the project within the newly started SFB 1141 Particulate Design, and is important for the application for SFB Catalysis at Liquid Interfaces.
- Z. Brkljača et int. P. Wasserscheid, et int., A-S. Smith. Complementary Molecular Dynamics and X-Ray Reflectivity Study of an Imidazolium-Based Ionic Liquid at a Neutral Sapphire Interface, J. Phys. Chem. Lett. 6 (2015) 549.
- A. Baer, et int., A-S. Smith, Water in an Electric Field does not Dance Alone: Relation Between Equilibrium Structure, Time Dependent Viscosity and Molecular Motions, J. Mol. Liquids 282 (2019) 303.
- R. Stepić, L. Jurković, K. Klementyeva, M. Ukrainczyk, M. Gredičak, D. M. Smith, D. Kralj, and A.-S. Smith, On the Adsorption of Aspartate Derivatives to Calcite Surfaces in Aqueous Environment. Cryst. Growth Des., 2020. DOI:10.1021/acs.cgd.0c00061.
- N. Vučemilović-Alagić, et int., A.-S. Smith, Insights from Molecular Dynamics Simulations on Structural Organization and Diffusion of an Ionic Liquid at Solid and Vacuum Interfaces, J. Colloid Inter-face Sci. 553 (2019) 350 and associated Data in Brief (DOI:10.1016/j.dib.2019.104794).
- T. Bauer et int., M. Haumann, P. Wasserscheid, A. Smith, J. Libuda, Dynamic Equilibria in Supported Ionic Liquid Phase (SILP) Catalysts: In-situ IR Spectroscopy Identifies [Ru(CO)xCly]n Species in Water Gas Shift Catalysis, Catal. Sci. Technol. 8 (2018) 344.
- R. Stepić, et int., M. Haumann, P. Wasserscheid, A.-S. Smith, D. M. Smith, Mechanism of the Water-Gas Shift Reaction Catalyzed by Efficient Ruthenium Based Catalysts, Angew. Chem. 14 (2019) 741.
- D. Blaumeiser, et int., M. Haumann, P. Wasserscheid, et int., A.-S. Smith, et int., J. Libuda. Cu Carbonyls Enhance the Performance of Ru-Based SILP Water-Gas Shift Catalysts: A Combined in-situ DRIFTS and DFT Study, Catal. Sci. Technol. 10 (2020) 252.
- P. Wolf, et int., A.-S. Smith, P. Wasserscheid, J. Libuda, M. Haumann, Improving the Performance of Supported Ionic Liquid Phase (SILP) catalysts for the Ultra-Low-Temperature, Green Chem. 21 (2019) 5008.
Researcher’s Bio and Affiliation
Nataša Vučemilović-Alagić obtained her master degree in Biophysics at Faculty of Science in Split, Croatia. Robert Stepić obtained his master degree in Theoretical Chemistry at Faculty of Science in Zagreb, Croatia while Andreas Baer graduated from the Elite Study Program of the Physics Department at FAU Erlangen. All three young researches are members of the PULS group of the Physics Department where they are working on their doctoral degrees under the supervision of Prof. Dr. Ana-Sunčana Smith. They are all enrolled in the Integrated Graduate School: Advanced Materials and Processes of the Excellence Cluster for Engineering of Advanced Materials. The work is performed in collaborations with several groups at FAU and with the Ruđer Bošković Institute, Zagreb,
Croatia.
